Two Canadian patients with spinal cord injuries have become the first outside the United States to get Neuralink chips — wireless devices implanted into their brains that allow them to control computers with only their thoughts.
É«É«Ŕ˛â€™s University Health Network announced the surgeries on Thursday, saying the successful procedures using Elon Musk’s Neuralink device mark a milestone in helping patients with paralysis.
The patients — both young men with cervical spinal cord injuries — recovered well after the implants and have quickly learned how to use the brain-computer interface, said UHN neurosurgeon Dr. Andres Lozano.
“Within a few minutes, the patient was able to learn how to move a cursor just by thinking about it,” said Lozano, who led the surgical team that performed the procedures at É«É«Ŕ˛ Western Hospital. The first patient received his Neuralink implant on Aug 27, while the second had the procedure on Wednesday.
“The first patient can now, for example, play video games,” Lozano said. “He can type by just thinking about moving from one character or keyword to another.

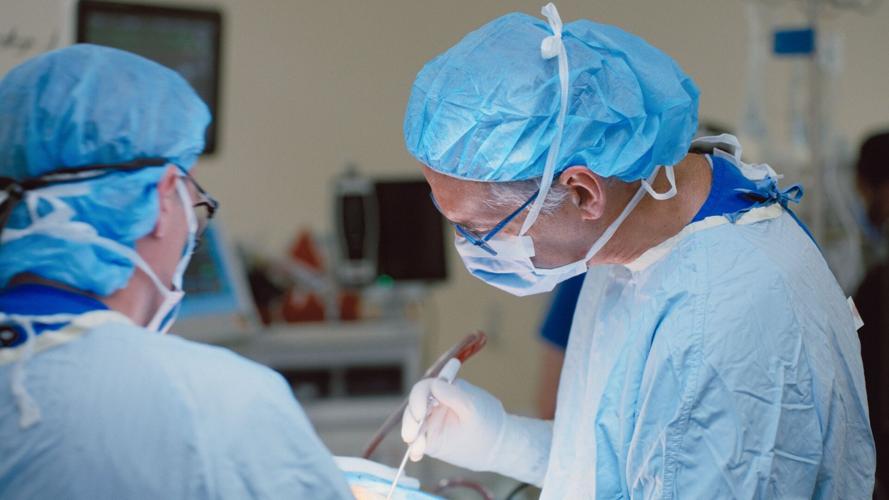
Dr. Andres Lozano, chair of neurosurgery at UHN, implants a Neuralink chip into the first Canadian patient to receive the computer-brain interface. The surgery was completed at É«É«Ŕ˛ Western Hospital on Aug. 27.Â
UHN/Neuralink“I was expecting it was going to be hard and laborious, that it was going to take a lot of training for him to be able to acquire this level of skill. But within minutes and without any instruction, he was just given the computer and a cursor, and he was able to figure this out.”
Neuralink is not the first or only brain-computer interface in development that holds promise to help people with paralysis move and communicate.
But the device grabs extra attention due to its links to Musk. The billionaire entrepreneur behind Tesla and SpaceXÂ has asserted that Neuralink will not only push boundaries in the medical world but may also provide superpowers to humans, giving people the same abilities as artificial intelligence.
The company announced its first Neuralink patient in early 2024. So far, 10 Americans and two Canadians have received a Neuralink implant, a UHN spokesperson said.
The company, which has received criticism for its lack of transparency around the development of its device, has also announced clinical trials in the United Kingdom and the United Arab Emirates.
The Canadian clinical trial at UHN, dubbed the CAN-PRIME Study, is expected to enrol six patients and will evaluate how well Neuralink’s implant — and its purpose-built robot required for the surgery — work and if they are safe. Neuralink received Health Canada approval for the trial last November.Â
Lozano said the hospital has four patients enrolled in the trial and is looking for more. People with a cervical spinal cord injury or amyotrophic lateral sclerosis (ALS) who have limited or no ability to use both hands may be eligible, he said. Â

 Dr. Andres Lozano, the Alan and Susan Hudson Cornerstone Chair in Neurosurgery at UHN.
UHN/NeuralinkThe first two Canadians to receive a Neuralink device were made aware of the risks, Lozano said, which include about a two per cent chance of severe complications, such as infection, stroke or suffering a cardiac event. He said both patients, who he described as “very keen and motivated,” were discharged from hospital within 24 hours of their surgeries.Â
“We think in the grand scheme of things, this surgery is relatively safe,” said Lozano, the Alan and Susan Hudson Cornerstone Chair in Neurosurgery at UHN.
The Neuralink device has more than 100 thread-like electrodes, each about one-fifth the diameter of a human hair, that get implanted into a patient’s brain, Lozano said. These threads have recording sites that “listen” to neurons as a patient thinks, Lozano said, adding that the interface component, accessed via Bluetooth, processes these signals, translating thoughts into action on an external device, such as a computer.
He said the Neuralink robot used during the surgery implants the electrodes in the right spot and depth, much like a “very precise sewing machine.”
The Canadian Neuralink patients will be followed for at least one year to determine whether the implant is safe and well-tolerated. Lozano said the team will also track how and when the patients use the device to see if it’s useful and to measure its impact on their lives.
At the end of the year, patients can choose to keep the implant or ask for it to be removed, Lozano said. He added that Neuralink engineers are available to help the patients with the brain-computer-interface and to troubleshoot if the device fails.
Lozano acknowledged there are big and unanswered ethical questions that surround Neuralink and other brain-computer interfaces, including their potential to augment human abilities.
“Right now, we’re talking about someone who’s disabled,” he said. “But it’s also possible, at least in theory, to take someone ... and upgrade their functions, whether this be memory or alertness. There is a whole slew of possibilities that we have to start thinking of in the future as to where this technology is going.”
Lozano said his team is focusing on whether the technology is safe and if it can improve the lives of patients with spinal injuries or neurological disorders.
For patients with ALS, for example, whose disease has progressed so far they are unable to move at all, including their eyes or mouth, Lozano said a brain-computer interface could be life-changing.
“It would be extremely liberating for a patient who is totally paralyzed and cannot communicate,” he said. “With this type of technology, it opens up a whole new world for them.”
  Â



























To join the conversation set a first and last name in your user profile.
Sign in or register for free to join the Conversation